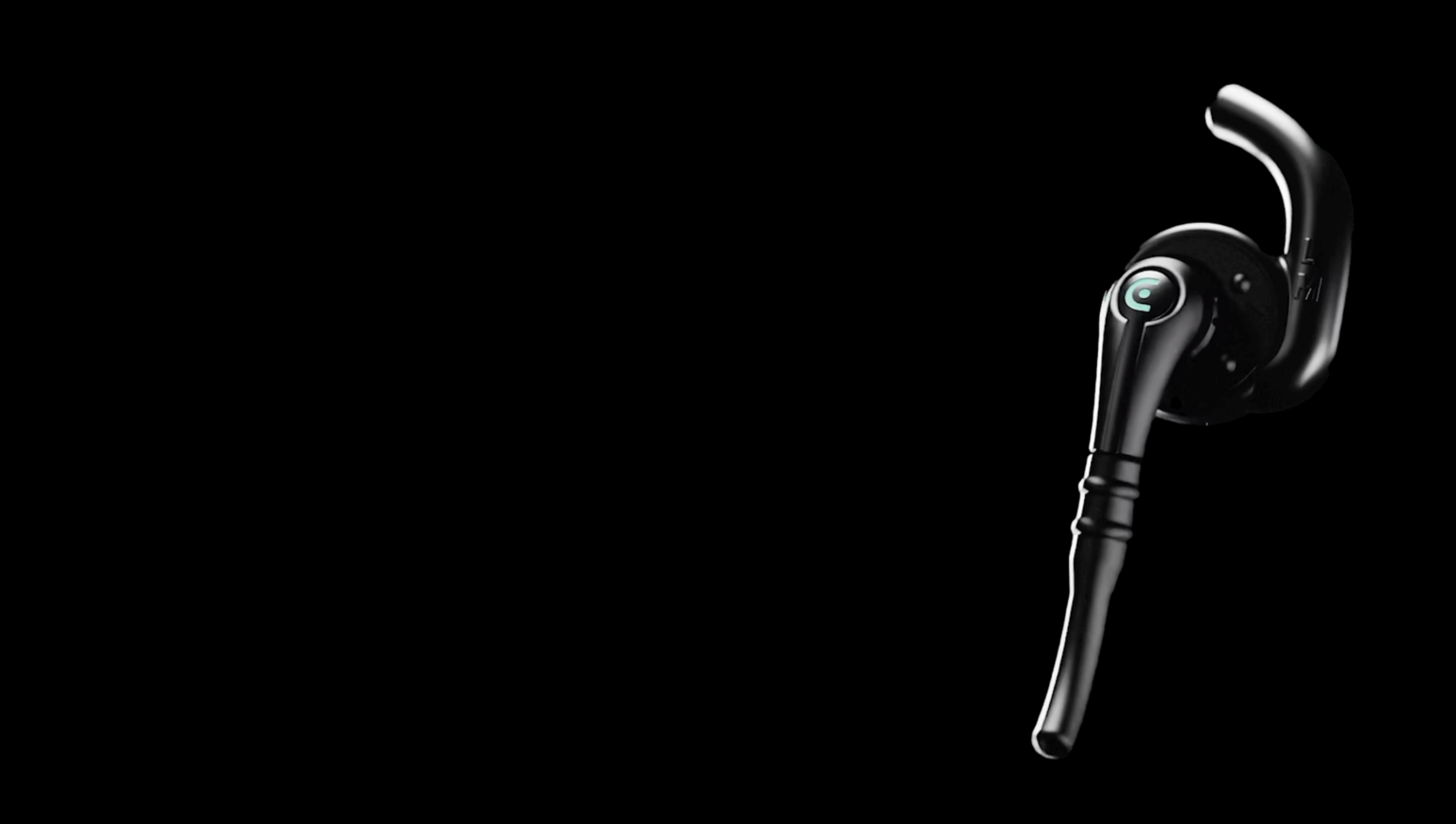
Digital health is increasingly being promoted as a key solution to the growing workforce and financial pressures faced by the NHS and healthcare systems around the world1.
For digital solutions to be truly effective and unbiased, it is essential that medical-grade diagnostics are developed and validated to be racially inclusive.
Worldwide, pulse oximetry is used in both clinical and home settings for routine monitoring, disease management, and remote care. By shining red and infrared light through the skin via a device clipped onto the fingertip, it is possible to measure blood oxygen levels and pulse rate.
Yet, following the COVID-19 pandemic, reports revealed that the accuracy of many fingertip oximeters is affected by skin colour – a bias that may have contributed to higher mortality rates among certain racial groups2.
In response, EarSwitch have introduced EarMetrics, which aims to transform everyday in-ear devices into racially inclusive medical monitors by capturing health data from a central site – the ear canal – where everyone’s skin colour is the same.
This inclusive technology aims to enable more accurate and equitable remote health monitoring. It also provides an opportunity to build large-scale, inclusive population health data – an essential foundation for the advancement of digital health.
Innovation
EarMetrics is a non-contact sensor that can detect multiple very detailed and fast-changing signals simultaneously. The sensor integrates seamlessly with everyday wearable devices, such as music earbuds or hearing aids, as well as in dedicated medical devices found in hospitals and GP surgeries.
For racially inclusive oximetry, an in-ear device uniquely targets a central site of the body where skin colour is consistent across all individuals. This core sensing site likely to be less affected by issues that hinder the accuracy of other sensor locations, such as limb movement, cold temperatures, poor circulation and of course, variations in skin tone.
By measuring different heart signals at the same time, EarMetrics can also monitor pulse transit time and pulse wave patterns. As such, the technology could potentially replace traditional cuff-based blood pressure measurements with a fully digital alternative.
Together, these capabilities enable the collection of standard clinical observations both in medical environments and during everyday life.
Moreover, the continuous and detailed data gathered through EarMetrics makes it possible to build a long-term picture of a person’s health supporting disease management, early screening, and the prediction of future health issues.
Finally, users will have the option to share their data, contributing to population-level insights that advance understanding and improve care for everyone.
WMHTIA support
EarSwitch have drawn on the clinical support of numerous WMHTIA partners, moving them closer to real world validation.
- Manufacturing Technology Centre (MTC): Technical assessment and product development to push the technology towards clinical trial readiness.
- University Hospitals Birmingham NHS Foundation Trust (UHB) / Medical Devices Testing and Evaluation Centre (MD-TEC): Funding obtained for first-in-human clinical investigation study through support to assemble a complete and compliant submission package, including all necessary clinical and technical data. Conducted preliminary feasibility assessments with University Hospitals Birmingham and engaged a lead clinical investigator.
- Medilink Midlands: Facilitation of connections with NIHR network.
- Plug and Play: Introductions to corporate partners, potential funding opportunities and commercial support. Wider networking opportunities provided during a US visit to Silicon Valley.
- WMHTIA grant recipient, utilising funding for additional testing and validation.
What’s next?
Extensive WMHTIA support and Asthma and Lung UK Charity funding have allowed the commencement of clinical validation of EarMetrics oximeter to achieve medical standards.
This pivotal traction will allow the company to progress to subsequent real world clinical validation in high risk intensive care patients.
EarSwitch are also continuing work with MTC for further technology advancement as well as, University of Leeds and Leeds Teaching Hospital NHS Trust to progress digital blood pressure monitoring (EarMetrics BP).
Nick Gompertz, Founder of Earswitch, said:
"The WMHTIA has provided funding, support, guidance and networking – globally and nationally; with this EarMetrics is much closer to becoming the new standard for safety critical healthcare all over the world.”
For more information on the work that EarSwitch are doing, visit www.earswitch.co.uk
The WMHTIA is part of the pilot Innovation Accelerator programme, which is led by Innovate UK on behalf of UK Research and Innovation and the Department for Science, Innovation and Technology.
This new model of funding focuses on locally-led innovation to drive economic growth and technological advancement by supporting regional innovative businesses, researchers and entrepreneurs. In the West Midlands, local leadership has been driven by a partnership comprising of the West Midlands Combined Authority, universities and other research institutions, and senior industry representatives.
Building on the £100 million already invested between 2022 and 2025, a further £30m was spread equally across three UK city-regions participating in the pilot Innovation Accelerator programme, which includes a funding boost of £4m for the WMHTIA to continue its support of Health Tech innovators in 2025/26.

Sources: